Additional information
| Active substance | Ampicillin |
|---|---|
| Water Retention | No significant effect |
| Hepatotoxicity | Rare, but hepatitis and cholestatic jaundice have been reported |
| Lab Test | No specific lab test required for monitoring ampicillin therapy |
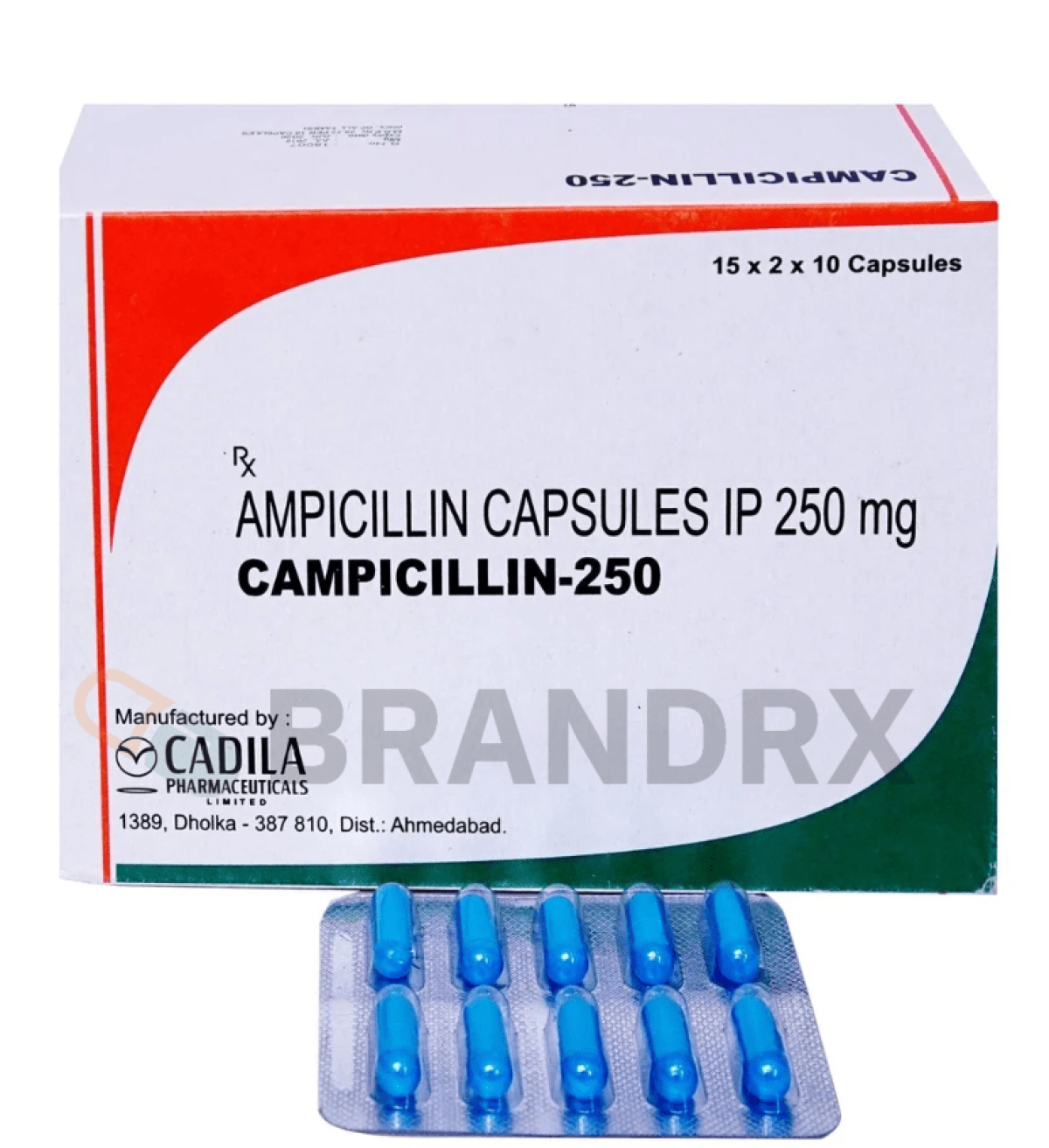
| Strength | 250mg |
| Also known as | Penbritin, Alpen |
| Blood pressure | No direct effect |
| Trade name | Principen, Omnipen, others |
| Storage conditions | Store at room temperature away from moisture and heat |
| Chemical name | (2S,5R,6R)-6-[(R)-2-Amino-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| Formula | C16H19N3O4S |
| Substance class | Antibiotic, specifically a beta-lactam |
| Main action | Bactericidal (kills bacteria by inhibiting cell wall synthesis) |
| Half-life | Approximately 1 hour |
| Dosage (medical) | Typically 250 mg to 500 mg every 6 hours orally, or 500 mg to 2 grams every 6 to 8 hours intravenously, depending on the type and severity of infection |
| Dosage (sports) | Not applicable |
| Effects | Effective against Gram-positive and some Gram-negative bacteria |
| Side effects | Rash, diarrhea, nausea, allergic reactions (including anaphylaxis), and potential superinfection |
| Use in sports | None |
| Manufacturer | Cadila Pharmaceuticals Ltd. |
| Packing | 10 caps/blister |






Reviews
There are no reviews yet.